08 2017 Aug 31, Novartis officially announced the FDA has approved the CAR-T cell therapy Kymriah (Tisagenlecleucel, CTL019) listed, for the treatment of refractory recurrent B cell precursor acute lymphoblastic leukemia (Acute lymphoblastic, leukemia, ALL), the drug had obtain breakthrough therapy designation and priority review.
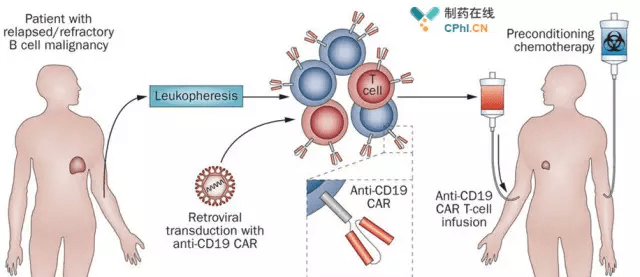
So far, Novartis global attention CAR-T cell products CTL019 finished the last mile of the listed Road, although this in 2017 07, 12 has no suspense, but still full of people looking forward to.
The approval is significant, although cellular immune therapy still has not overcome the limitations of their own, but the drug to critically ill patients to the last hope, I believe that the gene and cell therapy and other new technologies will be more mature and bring to human health more miracles.
Novartis CAR-T cell products approved the advantage in the competition with cellular immunotherapy with CAR-T cells, listed near the product pricing has become the focus of attention, how to give the product a reasonable price, worthy of attention.
In 2017 03, Jefferies analyst at Wall Streets famous investment bank pointed out that the price of CTL019 would go up to $600 thousand based on the price evaluation of clinical value (considering only young patients). The latest news, the blockbuster products may be priced 500 thousand dollars, Novartis using clinical pay scheme, but the scheme is considered to be a violation of the patient privacy but it has not been finalized, and finally discuss the institutions of the United states.
In addition, CAR-T cell production process, packaging, and transportation is a great challenge for research and development company will be faced with, news shows Cryoport will be CAR-T Kymriah therapy (CTL019) provides a low temperature logistics and transport support.
Novartis submitted a FDA Tisagenlecleucel (CTL-019) listing application for priority review, and the ELIANA clinical research (NCT02435849) is the core data supporting the application, with a surprisingly complete rate of remission.
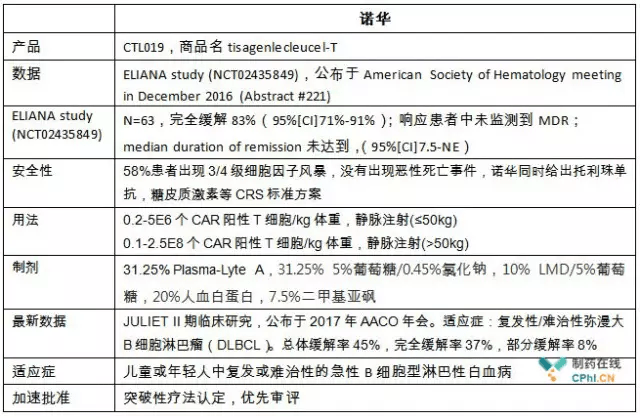
Of course, I expect Emily to be more fortunate, and even become a clinical norm. Now, although the CTL019 show an amazing effect, and is approved by FDA, but the scale of production of the long-term stability, toxicity, and market acceptance is the test of time, really get the recognition of patients, reduce the cost, benefit the patients become more mature commercial products, there is a long way to go.
Source: CPhI pharmaceuticals Online